Georg Cronheim published two articles in 1947 that give a thorough and intriguing description of the pharmaceutical uses of ozonides. Since 1947 remarkably few scientific discussions have been made with regard to the chemotherapeutic effects of ozonides, however, those that have been published seem very worthy of review. Changes in chemical nomenclature over the past 66 years require that I take a few sentences to clarify contemporary terms. Historically the term ozonide was used to describe any product or products that result from the ozonation of unsaturated compounds like waxes, rubbers, or oils. These "ozonides" were describing a mixture of ozonides, and or, hydroperoxides, and or, the many possible polymeric arrangements of each. As dozens of brilliant chemists, most notable, Christian Friedrich Schonbein, Carl Dietrich Harries, Rudolf Criegee, and Philip Bailey, have struggled to elucidate the true nature of ozonolysis, we now use the term ozonide to indicate a 1,2,4-trioxolane compound, as depicted in Figure 1.
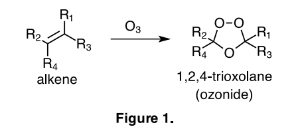
The production of the 1,2,4-trioxolane functionality is highly dependent on both the structure of the alkene being ozonated, and on the conditions of the reaction. Many alkenes will not produce 1,2,4-trioxolanes, as the major product, regardless of reaction conditions. This information is Impeccably described by Philip Bailey in his comprehensive review of ozone chemistry, which still stands as the preeminent reference for the reactions with ozone. For this discussion the term ozonide will refer to a compound with the 1,2,4- trioxolane functionality, sometimes described as the secondary ozonide in chemical literature, where the primary ozonide is the first adduct of ozone and the alkene. Thus an ozonide is distinguished from the complicated mixture of compounds produced by the reaction of ozone with unsaturated mixtures like oils, waxes, and rubbers.
Review
For those of you who don't remember the details of those 1947 journal articles I will reiterate a few of the more important references. The uses of ozonides described by Cronheim were mostly of topical preparations with germicidal properties, except the oral application for dogs and swine as described by Butz and La Lande. Inventors have been claiming dramatic medicinal uses for ozonides since 1902 when William Neel first described the medicinal use of ozonides for diseases of the blood and respiratory organs, where ozonated oils were inhaled. Then in 1917 William J. Knox ozonated ricinoleic acid or caster oil to produce a germicidal laxative. In 1921 James Todd published "Experiments with Oxygen on Disease" (Pittsburgh, PA) where he detailed the manufacture of ozonated olive, and cod-liver oils, that give "miraculous cures" with oral dosages. In 1937 Butz and La Lande successfully treated dogs infested with ascarides with oral dosages of an ozonide and diheptanol peroxide. They also found it of significance to note that no toxicity values could be determined for the ozonides or the diheptanol peroxide, as no toxicity was ever observed regardless of dosage. In 1942 Charles C. Johnson described the ozonation of the purified triglyceride 9 of oleic acid, and its fungicidal, germicidal, and deodorizing applications. Mr. Johnson also mentions “exceptionally meritorious results in treatment of secondary or tertiary burns”. Then after nearly half a century of dormancy, ozonides have reemerged by many orthogonal routes as this discussion will try to make clear. In 1986 De Villez used ozonated oils to treat acne. From 1988 to 1994 Stephen Herman generated a long list of patent publications, and a far longer list of claims for the uses of ozonides. The list of claims covers thousands of compounds, and hundreds of uses. I will list several of the uses described: treatment for insect stings, athletes foot, nail fungus, warts, viral infection, HIV, insecticide, fungicide, sunburn, serious burn repair, scar inhibitor, cancer, spermicide, arthritis, protozoal infection, leishmaniasis, to name most of the applications. In 1992 Luiz-Claudio de Almeida Barbosa et al. Describe accidentally isolating a stable ozonide with anti-malarial activity. Starting in the early 1990s a multinational team of chemists have synthesized, purified, and analyzed many dozens of ozonides with anti-malarial activity. Both in vitro and in vivo work is reported for both oral and subcutaneous dosages giving much insight as to the structure activity relationship (SAR). In his 2002 patent publication Vennerstrom et. al. described the synthesis, purification and physical properties of 90 spiro and dispiro 1,2,4 trioxolane compounds their thermal stability and the degree of anti-malarial activity of each. Vennerstrom also mentions in this document that these compounds have anti-cancer properties. The work from this group is extensive and beyond the scope of this review, but I would like to digress a moment to discuss the very high yields reported by Vennerstrom using fully substituted alkenes. In my opinion, both Creegee and Baily would be surprised at these results as their work points to very low yields for tetra substituted alkenes and much higher yields 1,2-substituted alkenes. In 1994 Davy K. Koech reports "Trioxolanes: a new generation of compounds with Wide Ranging Activities". And in 2008 Koech et al. published "Clinical 16 Applications of Trioxolane Derivatives" where he specifically indicates, an ozonide compound prepared and used effectively for the treatment of both AIDS and arthritis. In 1999 Dr. Gerhard Steidl published "The Fight Against Bacteria, Funguses, and Parasites by Supporting the Oxidative System in the Human Organism". This is the first of three papers published by Dr. Steidl concerning the use of ozonides. The second and third papers are titled, "Medicinal Microbiology Elimination of Pathogenic Bacteria, Fungi, Parasites, Viruses by Oxygen and Bitter Drugs" (Sept. 2000), and the more extensive follow on paper, "Use of Ozonides in the Treatment of Malignant Disease - basic principles and clinical results" (Jan. 2002). These documents seem to be web based publications found by a web search of the titles. Dr. Gerhard Steidls’ papers again describe hundreds of medical applications where virtually all known parasitic, viral, fungal, and tumor cells, can be treated, at least partially, as bitter drugs are used concomitantly with an ozonized oil. I would like to add that Dr. Steidl also suggests that some forms of depression, anxiety, hyperactivity, and hypo-activity may also be treated with these oxidative therapies. Sasaki et al claim anti tumor activity with an ozonide. In Sept. 2004 Hofmann et al describe the treatment of coronary arteriosclerosis by an oxidative therapeutic formulation, followed in April 2005 by a second patent describing the successful treatment of horses infected with sarcocystis protozoal infections. And a third patent issued in 2009 describes the bone regeneration 20 properties of these oxidative therapies. The most recent excitement surrounding the use of ozonides is related to the treatment of malaria. Most notable are the patents by Vennerstrom et al. describing antimalarial activity with dispiro-1,2,4-Trioxolanes.
Discussion
One must wonder why with the seemingly endless list of medical uses claimed by ozonides, with no significant side effects noted, why the market is relatively void of oxidative therapies. I shall propose several reasons why this might be the case however the true answer will likely be the complicated equilibrium between these and other unknown factors. First, the chemical literature is strewn with references to the explosive danger of trying to isolate trioxolane molecules. I must confess to my excessive care (fear) when first working with these compounds as I understood from much of the chemical literature that ozonides were very unstable, and subject to explosive decomposition. Ozonides are traditionally thought of as only intermediates in ozonolysis, where an alkenes is converted to; alcohols, aldehydes, ketones, carboxylic acids, or peroxides, and the isolation of the ozonide has been strictly avoided by much of chemical literature. I would like to support this statement with a few quotes, wherein ozonides are specifically discouraged from being isolated: From the 1966 US patent 3,284,492, where Fremery and Fields state: "Ideally the highly reactive and unstable ozonide should be converted quickly and simply to the desired product.” From the current Organic-Chemistry.org description of ozonolysis, "while secondary ozonides are more stable than primary ozonides they should not be isolated from an unmodified ozonolysis as other explosive compounds may have been formed". And this online article written by Derek Lowe, indicated the current thinking of most pharmaceutical chemists, where Dr. Lowe questions the sanity of trying to develop an ozonide for use as a drug, as he states, “there are some structures that I just wouldn’t make on purpose, and which I wouldn’t submit for testing even if I made them by accident.” As far as the explosive reputation of ozonides, I think a few points from Philip Bailey could go a long way to clear up the misconception about their stability, “...most of the explosive “ozonides” of early literature were not ozonides at all, but polymeric peroxides, or ozonides contaminated with such.” Bailey goes on to 25 describe how many ozonides have been safely isolated, and that discrete melting points of over 100 Centigrade is not uncommon. Clearly, the extensive work performed by Vennerstrom, and what must be a small army of other excellent chemists, clearly demonstrate the stability of these compounds as most decompose between 150-170C. Second, It may be that the continued bombardment by product advertisers about the benefits of "antioxidants" has, indirectly, given a bad reputation to oxidizing compounds. In fact, the opposite seems true, as I can think of many examples where peroxides and ozonides demonstrate healing properties, and can think of no therapeutic reducing agents. It has also been suggested that the technology of oxidative therapeutic treatments were abandoned as a result of the discovery of “modern” antibiotics, as might be corroborated by the disappearance of ozonide research after 1947.
Summary
It seems clear that aerobic biology of eukaryotic organisms is very compatible with oxygen and reactive oxygen species (ROS) like singlet oxygen, superoxide, and other oxygen radicals. Whereas anaerobic organisms (prokaryote) like bacterium, and fungus, do not generally tolerate these type of oxygen species. It also seems obvious that there are many pharmaceutical opportunities for the use of ozonides as antimicrobial agents. Lastly, it has been suggested that the technology of oxidative therapeutic treatments were abandoned as a result of the discovery of “modern” antibiotics. It's also has been suggested that our understanding of modern antibiotics is not perfect, and that they are a very poor treatment for parasitic and fungal infestation, is a good reason for reevaluation of the “early” chemical research. Let me end with a quote from my esteemed predecessor, "The pharmaceutical use of oxygen releasing compounds dates back to 1818 when Thenard discovered hydrogen peroxide. The compounds that have been employed since then are mostly inorganic and organic peroxides. Very little attention has paid to ozonides which can also decompose to release nascent oxygen and can be used in the same manner as peroxides." Georg Cronheim, 1947
Ross Herman, Ph.D.
References (22)
- Cronheim, G. Organic Ozonides as Chemotherapeutic Agents, l. Chemical 1 studies. J. Pharm. Sci., 36: 274-278.
- Cronheim, G. Organic Ozonides as Chemotherapeutic Agents, lI. Antiseptic 2 properties. J. Pharm. Sci., 36: 278-281.
- Criegee, Rudolf. Mechanism of Ozonolysis. Chem. Int. Ed. Engl. 3 14(11): 745-752.
- Bailey, P. S. Ozonation in organic chemistry, Volume 1 olefinic compounds. Academic Press, New York (1978).
- Cronheim, G. Organic Ozonides as Chemotherapeutic Agents, l. Chemical 5 studies. J. Pharm. Sci., 36: 274-278.
- Neel, W. D. US patent 925590 (July 1902).
- Knox, W. J. US patent No. 1,210,949 (Jan 1917).
- Butz, L. W. and La Lande, W. A. Anthelmintics II. A comparison of certain ozonides, 8 chenopodium oil and diheptanol peroxide. J. Pharm. Sci., 26: 114–121. doi: 10.1002/jps.3080260206.
- Johnson, C. C. US patent 2,356,062. (Aug. 1944) 9 10 De Villez, US4451480 and US4591602.
- Herman, Stephen. EP0427781 A4, EP0476054 A4, US4983637, US5086076, 11 US5093326, US5126376, US5190977, US5190979, US5260342, US5270344, and US5364879.
- Luiz-Claudio de Almeida Barbosa et al. J. Chem. Soc. Perkin Trans. 1, 1992, pp 12 3251-3252.
- Luiz-Claudio de Almeida Barbosa et al. J. Chem. Soc. Perkin Trans. 1, 1996, pp 13 1101-1105.
- Vennerstrom et al. US patent 6,486,199 B1 (Nov. 26, 2002).
- Koech et. al. Trioxolanes: A New Generation of Compounds with Wide Ranging Activities. Afr. J Health Sci. Vol. 1 No. 4 (Nov. 1994).
- Koech, D. K. Clinical applications of trioxolane derivatives. Afr. J Health Sci., Vol 16 15, No. 1-2, pp 1-5, 2008.
- 17 US patent 6365610 B1, April 2002.
- Hofmann et al. US patent 6790463 B2 (sept 2004).
- Hofmann et al. US patent 6,790,463 B2 (April 2005).
- Hofmann et al. US patent 7,572,782 B2 (Aug. 2009).
- Valecha, N, et al. Clin Infect Dis. Vol. 51, issue 6, pp 684-691, Sept 2010.
- Uhlemann et al. Mechanisms of 22 Antimalarial Action of the Synthetic Trioxolane RBX11160. Anitmicrobial Agents and Chemotherapy Vol. 51, issue 2, pp 667-672 (Feb 2007).
- Vennerstrom et al. US patent 6,486,199 (Nov 2002) and US 8,067,620 B2 (Nov 23 2011).
- Bailey, P. S. Ozonation in organic chemistry. Volume 1 olefinic compounds. 25 Academic Press, New York (1978).
†Results may vary. Information and statements made are for education purposes and are not intended to replace the advice of your doctor. If you have a severe medical condition or health concern, see your physician.







